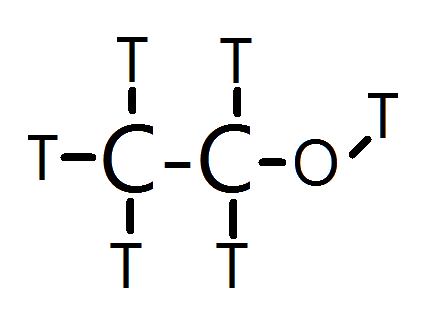Plasma: различия между версиями
Iishandry (обсуждение | вклад) м (Типаперевол.) |
Feomatar (обсуждение | вклад) (Начал переводить, завтра продолжу.) |
||
| Строка 4: | Строка 4: | ||
=The States of Plasma= | =The States of Plasma= | ||
| − | + | Плазма может встречаться в разных формах. | |
| − | *'''Plasma Ore''' | + | *'''Плазменная Руда (''Plasma Ore'')''' |
| − | ** | + | **Её добывают [[Shaft Miner|шахтёры]] на шахтёрской станции. |
| − | *'''Solid Plasma''' | + | *'''Твёрдая плазма (''Solid Plasma'')''' |
| − | **'' | + | **''Плазменную Руду'' можно переплавить в куски Твёрдой Плазмы в специальной плавильне в Карго. |
| − | *'''Liquefied Plasma''' | + | *'''Жидкая Плазма (''Liquefied Plasma'')''' |
| − | ** | + | **Измельчители для реагентов подойдут, чтобы выжать из кусков плазмы сок. |
| − | *'''Plasma Gas''' | + | *'''Газированная Плазма (''Plasma Gas'')''' |
| − | ** | + | **В начале раунда находится в Атмосферном отсеке, у Инженеров и в РнД. Получить её другим способом нельзя. |
| − | = | + | =Где Используется= |
| − | *[[Guide_to_chemistry| | + | *[[Guide_to_chemistry|Как реагент в химических реакциях]]. |
| − | * | + | *Проявлять свойства экстрактов в [[Guide to Xenobiology|Ксенобиологии]]. |
| − | * | + | *Вести исследование по [[Virologist|вирусологии]]. |
| − | * | + | *Делать бомбы. |
| − | *[[Guide_to_food_and_drinks#Drinks| | + | *[[Guide_to_food_and_drinks#Drinks|Отравлять]] людей. |
| − | * | + | *Топливо для P.A.C.M.A.N. |
| − | * | + | *Может пригодиться инженерам, для сингулярного двигателя. |
| − | * | + | *Получить больше очков заказов для карго-шаттла. |
| − | *[[ | + | *[[Guide to Atmospherics|Сжечь]] всё к чертям]. |
| + | =Анализ: Суть Плазмы= | ||
| + | И так, некоторое время NT занимались изучением плазмы. Однако, наняли для этого каких-то пироманов и террористов. Так-что что это такое толком ничего не известно, кроме как то, что это розовая хрень, которая неплохо горит. | ||
| − | + | Так вот, когда углеводород горит, он производит CO<sub>2</sub> и H<sub>2</sub>0. '''Горящая плазма производит ТОЛЬКО CO<sub>2</sub>.''' Из этого следует, что Плазма это углеводород, и состоит ТОЛЬКО из водорода и углевода. ВОЗМОЖНО, в ней есть ещё и кислород, но это спорно. Учитывая что в SS13 нет воды как газа, можете отбросить ту часть про воду. И ещё одно про углеводороды - они ведь обычно газы, и такие как Гексан, быстро испаряются. (Так-что не стоит ронять ту зловещую пробирку). | |
| − | |||
| − | + | ===А теперь, немного чисел и сухой информации=== | |
| − | |||
| − | |||
| − | |||
From here on in, a CANISTER refers to to the large, colored gas containers that you fill TANKS in. A CANISTER needs to be pulled, a TANK can be held in your hand. | From here on in, a CANISTER refers to to the large, colored gas containers that you fill TANKS in. A CANISTER needs to be pulled, a TANK can be held in your hand. | ||
Версия от 22:44, 11 ноября 2015
|
Нужен перевод |
| Данная статья помечена как не переведенная. Это означает, что информация в статье верна, но требует перевода с иностранного языка. Помните! Часто помимо знания языка требуются специализированные знания в некоторых областях. Если не уверены, что это переводится именно так—не переводите. |
Чудесное вещество, одно из тех, что Станции 13-ой модели добывают и изучают.
The States of Plasma
Плазма может встречаться в разных формах.
- Плазменная Руда (Plasma Ore)
- Её добывают шахтёры на шахтёрской станции.
- Твёрдая плазма (Solid Plasma)
- Плазменную Руду можно переплавить в куски Твёрдой Плазмы в специальной плавильне в Карго.
- Жидкая Плазма (Liquefied Plasma)
- Измельчители для реагентов подойдут, чтобы выжать из кусков плазмы сок.
- Газированная Плазма (Plasma Gas)
- В начале раунда находится в Атмосферном отсеке, у Инженеров и в РнД. Получить её другим способом нельзя.
Где Используется
- Как реагент в химических реакциях.
- Проявлять свойства экстрактов в Ксенобиологии.
- Вести исследование по вирусологии.
- Делать бомбы.
- Отравлять людей.
- Топливо для P.A.C.M.A.N.
- Может пригодиться инженерам, для сингулярного двигателя.
- Получить больше очков заказов для карго-шаттла.
- Сжечь всё к чертям].
Анализ: Суть Плазмы
И так, некоторое время NT занимались изучением плазмы. Однако, наняли для этого каких-то пироманов и террористов. Так-что что это такое толком ничего не известно, кроме как то, что это розовая хрень, которая неплохо горит.
Так вот, когда углеводород горит, он производит CO2 и H20. Горящая плазма производит ТОЛЬКО CO2. Из этого следует, что Плазма это углеводород, и состоит ТОЛЬКО из водорода и углевода. ВОЗМОЖНО, в ней есть ещё и кислород, но это спорно. Учитывая что в SS13 нет воды как газа, можете отбросить ту часть про воду. И ещё одно про углеводороды - они ведь обычно газы, и такие как Гексан, быстро испаряются. (Так-что не стоит ронять ту зловещую пробирку).
А теперь, немного чисел и сухой информации
From here on in, a CANISTER refers to to the large, colored gas containers that you fill TANKS in. A CANISTER needs to be pulled, a TANK can be held in your hand.
A full canister of plasma has an internal pressure of 4559.6 kPa. Said full canister of plasma was released in a 6x6 room, built in space. This room was entirely devoid of air, and had a temperature of 0 Celsius (270 Kelvin). A gas analyzer was used to measure the pressure in this 6x6 room. It was 20.4 kPa, and the temperature was 19C (289K). NOW, HERE IS THE VARIABLE PART: In ooc, it was agreed upon that a tile had a volume of 1.6 m3. HOWEVER. In the code, it says that a gas cell is 2.5m3. Ultimately, using the 2.5 m³ tile messes things up, getting us into a situation where we end up with half a carbon, and, yeah. For the sake of completion, I'll post the math for both.
First, the 1.6 m³:
Using the Ideal Gas Formula (PV=nrt), we can calculate the number of moles of plasma in our room, via some algebra fandangling. PV=nrt/rt = (PV)/(rt)=n. Pressure times volume DIVIDED BY gas constant times temperature equals moles.
1.6 m³ = 4.096. 6x6 = 36. 4.096 x 36 = 147.456. 1 m³ = 1000 l. 147.456 x 1000 = 147,456 l.
(20.4 kPa)(147,456 l) / (8.314 kPa/l/K/mol)(292 K) = 3008102.4 / 2402.746 = 1251.95 mol plasma.
NOW FOR THE 2.5 m³:
2.5³ = 15.625 x 36 = 562.5 x 1000 = 562,500 l
Plugging the 562,500 l into the equation and replacing the 147,456 l with it gives us 4726.8 mol of plasma.
This is quite a difference.
((((I also did this for oxygen, but ultimately that data is not needed for this, so yeah)))))
NOW ONTO THE PART THAT INVOLVES FIRE
A full tank of plasma, which contains either 4774.80 mol or 1251.95 mol of plasma at 4559.6 kPa, was pumped into the incinerator burn chamber. An excess of oxygen was then pumped in, and the whole mix was ignited. After it cooled, our brave atmos tech scientist entered. Some time was taken to let the mixture spread throughout the 10 tile area. The area was then scanned.
375.35 kPa, at 97.4 °C (370.4K). THIS WAS DONE ON ASTEROIDSTATION HOWEVER, and on Asteroid, the incinerator burn chamber is not empty! CO2 53 %, O2 36 %, N2 9 %.
375.35 x 0.53 = 198.935 kPa.
Go CO2 woohooo
Now let's see how much CO2 a single canister of plasma can make. 156,250 l for the volume of the 2.5 m³.
10 x 4.096 x 1000 = 40,960 l
10 x 15.625 x 1000 = 156,250 l
(198.935)(40,960 l) / (8.314)(370.4) = 2,646 mol CO2.
(198.935)(156,250 l) / (8.314)(370.4) = 10,093.696 mol CO2.
EVERYTHING IN ITALICS IS CORRECT AS FAR AS PROCEDURE AND METHOD GOES, BUT THE ACTUAL NUMBERS ARE INCORRECT. I'M ONLY KEEPING IT AS AN EXPLANATION OF CONVERTING MOLES OF CO2 TO MOLES OF CARBON.
Now then, because moles are hilarious little units, if we have 591.17 mol of CO2, we also have 591.17 mole of C.
591.13 mol / (1 molC / 1 molCO2) = 591.13. Don't believe me? 591.13 mol / 1 molCO2 x 46 = 27,192 g. The molecular weights of Carbon and CO2 are 14 and 46 respectively. The percent by mass of carbon in CO2 is 14 / 46 = 0.3043 x 100 = 30.43 %. 27,192 x 0.3043 = 8274.52 g carbon. 8274.52 g carbon / 14 gC x 1 molC = 591.13 molC. Fucking math, how does it work. The same applies for the 2.5 m³ one. So it's 2232 mol Carbon if a tile is 2.5 m³.
More CO2
Now, That means that 1251.94 molPlasma + ??? molO2 ----> ??? molH2O + 2,656 molCO2. But because we did the above, we can basically summarize that there are 2,646 mol of carbon in 1251.94 mol of Plasma.
Pure hydrocarbon chains exhibit a funny property. By taking the amount of CO2 produced (and thus, carbon), and dividing it by the moles of hydrocarbon you burnt to get said CO2 (and assuming you have 100 % total combustion, no carbon monoxide produced), you can find out how many carbons are in a single mole of the Hydrocarbon!
Thus, the burning of Hexane is:
C6H14 + (19/2)O2 ---> 6CO2 + 7H20
So:
12C6H14 + blah --> 72CO2 + blah
72 / 12 = 6 !
THUUUUUUUS!
2656 / 1251.95 If we divide the two, we get 2.11, meaning that for every 1 mole of plasma, we have 2.11 moles of carbon!
And if we use the 2.5 m³, we end up with 10,093 / 4995 = 2.02! Told you it didn't matter.
Now, this is all theoretical, and calculations, and I've rounded a lot of the decimals (I ain't working with 24.858849389200102299383838392929292 due to spessmens, 2.858 will suffice), we can thus say, that there are TWO CARBONS IN A PLASMA MOLECULE. Plus, y'know, you can't have .11, or .02, of a molecule. Shit just ain't possible.
Which means that plasma has a structure that looks something like:
C-C,,,,C=C, or C≡C
With three, two, and one bonding site on each carbon, respectively.
A rant about isotopes
But...Then plasma is just a regular derivative of ethane, ethene, or ethyne! "what the fuck pybro you're such a fucking faggot you waste my very precious ten minutes!". Hold up there! Yes, it's just regular ethene, ethane, IF YOU USE 1H, AKA Protium, AKA "Normal hydrogen" AKA "A single proton with an electron buzzing around it". Atoms are composed of protons (positive), electrons (negative), and neutrons (neutral). Isotopes are atoms that have a "deviant" number of neutrons. This messes shit up, and gives isotopes different properties than the "normal" element. Generally (although there are exceptions!), the "normal" isotope has as many neutrons as it does protons. Protium AKA "normal" hydrogen, is one such exception.
Hyrogen has two isotopes. One, Deuterium, which is stable, and forms Deuterium Oxide (D2O), AKA "Heavy Water" (heavy water icecubes don't float on top of liquid water or heavy water, fun fact). Deuterium is a proton, a neutron, and an electron. There is also, TRITIUM, which is two neutrons, a proton, an electron. Tritium is also radioactive. On earth, Tritium is rare. In spess however, it's rather common (the source of tritium on earth is normal hydrogen getting hit by COSMIC RAYS). Tritium is also very toxic.
Hydrogens isotopes are very special, in that they get their own letters! Protium is H, Deuterium is D, and Tritium is T.
I propose that the hydrogens in plasma are actually Tritium. This raises the molecular mass up to the point where you could have a beaker of it as a liquid, but upon spilling it, get a gas. Tritium is toxic, but only when you actually get it in you, despite the fact that it's radioactive, the radiation from it is not strong enough to break your skin, although it might give you skin cancer, but your guts will be fine.
Thus, we know the following:
- Plasma is a two carbon compound, and the only two other elements that are/could be in it are hydrogen and oxygen.
- Plasma is a volatile liquid.
- Plasma can be a liquid, gas, and solid at room temperature.
- Plasma is highly toxic when inhaled, drank, and injected, but is fairly harmless (in the short term) when your skin touches it. Unless, y'know, you get set on fire.
- Plasma burns readily in the presence of oxygen, both when a liquid and a gas.
- When mixed with nitrogen and hydrogen, it produces lexorin, which basically shuts down cellular respiration. While not as potent as raw cyanide, lexorin is most certainly a brand name of some nitrile compound that NanoTrasen has a trademark on and sells as a paint thinner or something. BUT! Cyanide/Nitrile is basically a carbon and a nitrogen triple bonded together, and lexorin is made by adding plasma, nitrogen, and hydrogen.
Thus, I propose that Plasma looks something like this:
Essentially, Tritiated Ethanol. It's volatile, burns very easily, the Alcohol group at the right (Tritahol?) provides it with enough mass and hydrogen bonding (tritium bonding?) to keep it stable as a liquid, yet is still volatile, and is incredibly bad to drink.
This also answers several questions.
One, plasma is a radioactive compound, yes, but the halflife of Tritium is 12.3 YEARS. It is very fucking toxic, and is a great catalyst/solvent. It's not very toxic ON CONTACT, for short periods. But upon ingestion, it basically fucks your whole body up. The biochemistry of this is that your body uses the tritium/tritium oxide to replace your normal protium. Now, Deuterium in large quantities fucks you up because it's heavier than protium, and as such, has interesting effects on your body (and by interesting I mean "bad/deadly"). Now let's make it even heavier, and radioactive. Yeah.
Two, plasma would be rare on Earth, but in a gas giant that's composed mainly of this shit, it'd be produced easily. Even more so if the Plasma-Gas-Giant doesn't have an atmosphere that could block out the cosmic rays.
Three, Tritium has a rather large half life, and even then is rather weak as far as radioactivity goes (you still don't want it in you, however). You can dunk yourself in tritium oxide (T2O), and so long as you don't get any IN YOU, your guts will be fine, you only risk skin cancer.
Four, given that burning plasma only produces CO2 (and water, which is actually Tritium Oxide, aka super-heavy water, aka radio-fucking-active water), it can ONLY have Hydrogen (or isotopes of), Carbon, and oxygen in it. Tritium is necessary to get the weight up into the range where it can be a liquid at standard temperature and pressure AT ALL, while at the same time giving it the necessary toxicity. Plasma gives you TOXIN damage, it does NOT give you oxyloss damage, so it doesn't just suffocate you, it actively attacks your body in SOME way. Ethane (C2H6) does not do this, and is pretty much completely non-toxic. Hell, the only real dangers of ethane are asphyxiation (oxyloss) and igniting it (burn/bomb).
Five, the OH (or OT, as it were) is necessary to also bump up the weight into the range where it can be a liquid/solid at room temperature. It also gives it enough intermolecular forces (the hydrogen/tritium bonding) to aid it being a liquid.
Six, Solid plasma is plasma put under intense pressure for a quick moment, allowing it to crystallize. When left alone, it will eventually melt, and evaporate.
Seven, NOWHERE IS THE EXPLICIT MASS OF PLASMA ACTUALLY STATED.
Eight, the combustion equation for plasma would be:
C2T5OT + 3O2 ---> 2CO2 + 3T2O
Theory by Pybro